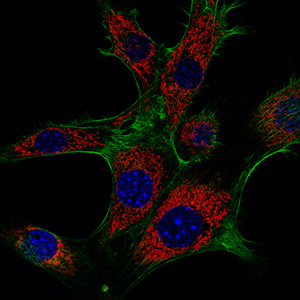
Actin cytoskeleton
Formin proteins contribute to a variety of different cellular actin cytoskeleton structures by their ability to polymerize straight actin filaments at the barbed end. Formins are able to cluster and associate at the plasma membrane while elongating actin filaments at the leading edge. A subgroup of mammalian formins, termed Diaphanous-related formins or DRFs, is activated by small GTPases of the Rho superfamily. DRFs are auto-inhibited in the resting state by an N- to C-terminal interaction that renders the central actin polymerization domain inactive. Upon the interaction with a GTP-bound Rho, Rac or Cdc42 GTPase, the C-terminal autoregulation domain is displaced from its N-terminal recognition site and the formin becomes active to polymerize actin filaments. We are interested in the structure, activation, and function of formins and their interaction with cellular co-factors. The multifaceted function of formins as effector proteins of Rho GTPases reflects the diversity of the actin cytoskeleton in cells.
Selected reading
- Grueb, S.S., et al. (2019). The formin Drosophila homologue of Diaphanous2 (Diaph2) controls microtubule dynamics in colorectal cancer cells independent of its FH2-domain. Sci. Rep. 9:5352.
- Kage, F., et al. (2017). FMNL formins boost lamellipodial force generation. Nat. Commun. 8:14832.
- Kühn, S., et al. (2015). The structure of FMNL2–Cdc42 yields insights into the mechanism of lamellipodia and filopodia formation. Nat. Commun. 6:7088.
- Kühn, S. and Geyer, M. (2014). Formins as effector proteins of Rho GTPases. Small GTPases 5, e29513.
- Schulze, N., et al. (2014). FHOD1 regulates stress fiber organization by controlling the dynamics of transverse arcs and dorsal fibers. J. Cell Sci. 127, 1379–1393.
- Schönichen, A., et al. (2013). FHOD1 is a combined actin filament capping and bundling factor that selectively associates with actin arcs and stress fibers. J. Cell Sci. 126, 1891–1901.


