Membrane proteins
Every living cell is surrounded by at least one lipid bilayer. This thin membrane is about 4 nm thick and enables the cell to establish a carefully balanced composition of nucleic acids, proteins, cofactors, nutrients and ions. At the same time, traffic of molecules and information accross this barrier must be controlled. This task is performed by membrane proteins. In humans, 25% of all protein coding genes encode membrane proteins. Many of them are important drug targets.
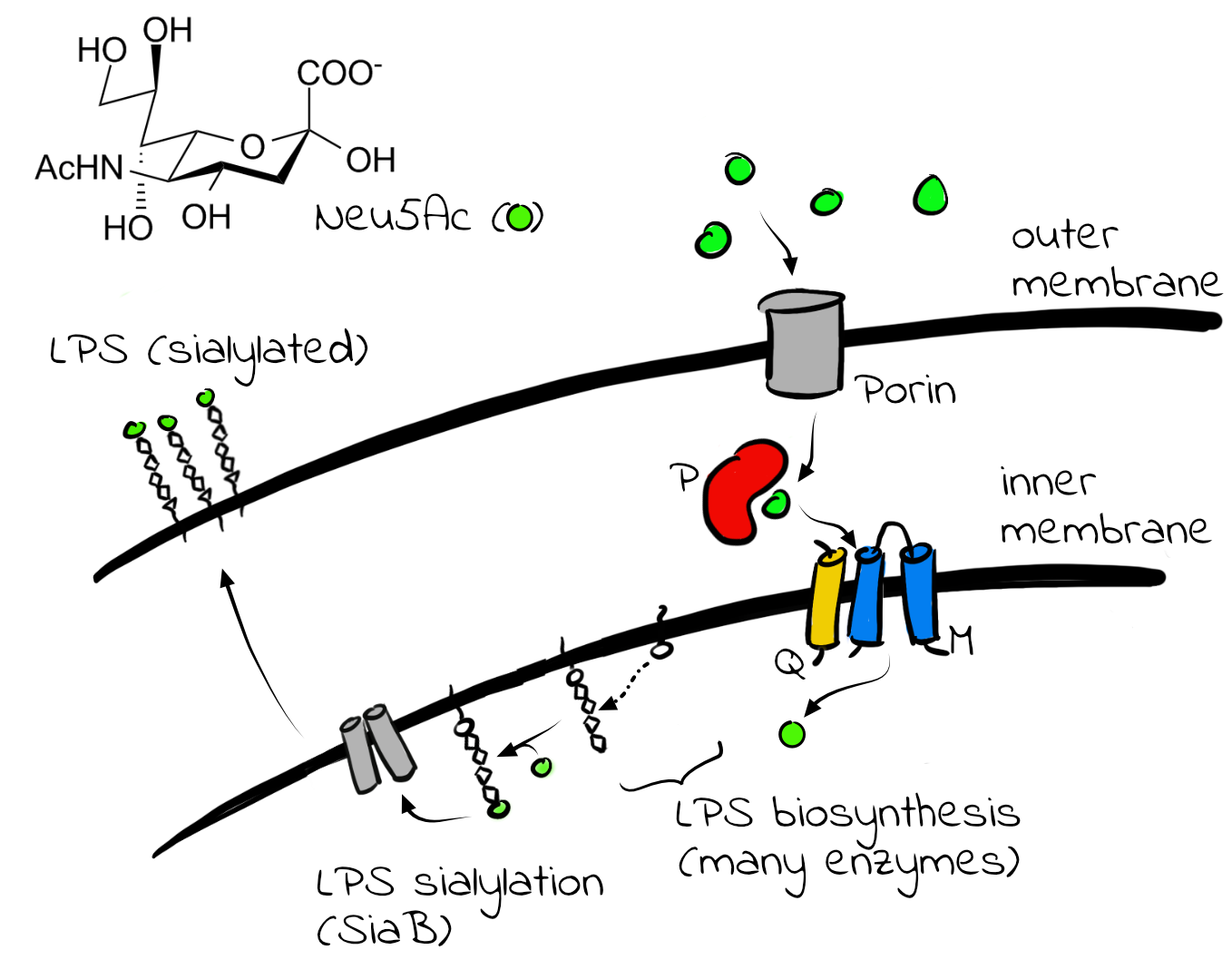
Among the many biological processes that are controlled by membrane proteins is the decoration of bacterial lipopolysaccharides (LPS) with sialic acid. Bacterial lipopolysaccharides (LPS) are highly diverse carbohydrate structures that are very strong activators of the human innate immune system. Some pathogens such as Haemophilus influenzae attach sialic acid molecules (also known as Neu5Ac) to their LPS, as a strategy to avoid or to dampen the innate immune response. They employ so called tripartite ATP-independent periplasmic (TRAP) transporters to scavenge the sialic acid from host tissues.
TRAP transporters are not present in humans and are thus not only interesting from a biochemical perspective, but also as potential drug targets. They consist of three subunits that are commonly called P, Q and M. The P domain collects the substrate in the periplasm and shuttles it to the transporter subunits (Q- and M). We employ X-ray crystallography and cryo-EM combined with biophysical techniques to determine the structure and function of the different TRAP transporter domains.
Among the many biological processes that are controlled by membrane proteins is the decoration of bacterial lipopolysaccharides (LPS) with sialic acid. Bacterial lipopolysaccharides (LPS) are highly diverse carbohydrate structures that are very strong activators of the human innate immune system. Some pathogens such as Haemophilus influenzae attach sialic acid molecules (also known as Neu5Ac) to their LPS, as a strategy to avoid or to dampen the innate immune response. They employ so called tripartite ATP-independent periplasmic (TRAP) transporters to scavenge the sialic acid from host tissues.
TRAP transporters are not present in humans and are thus not only interesting from a biochemical perspective, but also as potential drug targets. They consist of three subunits that are commonly called P, Q and M. The P domain collects the substrate in the periplasm and shuttles it to the transporter subunits (Q- and M). We employ X-ray crystallography and cryo-EM combined with biophysical techniques to determine the structure and function of the different TRAP transporter domains.
Selected reading
- M.F. Peter et al., "Structural and mechanistic analysis of a tripartite ATP-independent periplasmic TRAP transporter", 2022, Nat. Commun. 13:4471 doi:10.1038/s41467-022-31907-y
- R. Rabus, D.L. Jack, D.J. Kelly, M.H. Saier, "TRAP transporters: an ancient family of extracytoplasmic solute-receptor-dependent secondary active transporters", 1999, Microbiology, 145, 3431-3445
- C. Mulligan, M. Fischer, G.H. Thomas, "Tripartite ATP-independent periplasmic (TRAP) transporters in bacteria and archaea", 2011, FEMS Microbiol. Rev., 35, 68-86


