Together with Sneha Singh, Arijit Biswas and Johannes Oldenburg from the Institute of Hematology, Gregor and Matthias determined the first structure of the A and B subunits of Factor XIII. This tetrameric protein complex plays an important role in blood coagulation and is linked to many bleeding disorders. Using single particle cryo-EM, we were able to resolve the FXIII-A2B2 complex at 2.4 Å resolution. Read all about this exciting story here.
Congratulations to Jan Gerhartz, Dominika Pieńkowska, Ina Dressel and Thomas Geiger on a paper in Cell Chemical Biology.
An engineered cereblon optimized for high throughput screening and molecular glue discovery - now in Cell Chemical Biology. Congratulations Jan Gerhartz, Dominika Pieńkowska, Ina Dressel and Thomas Geiger.
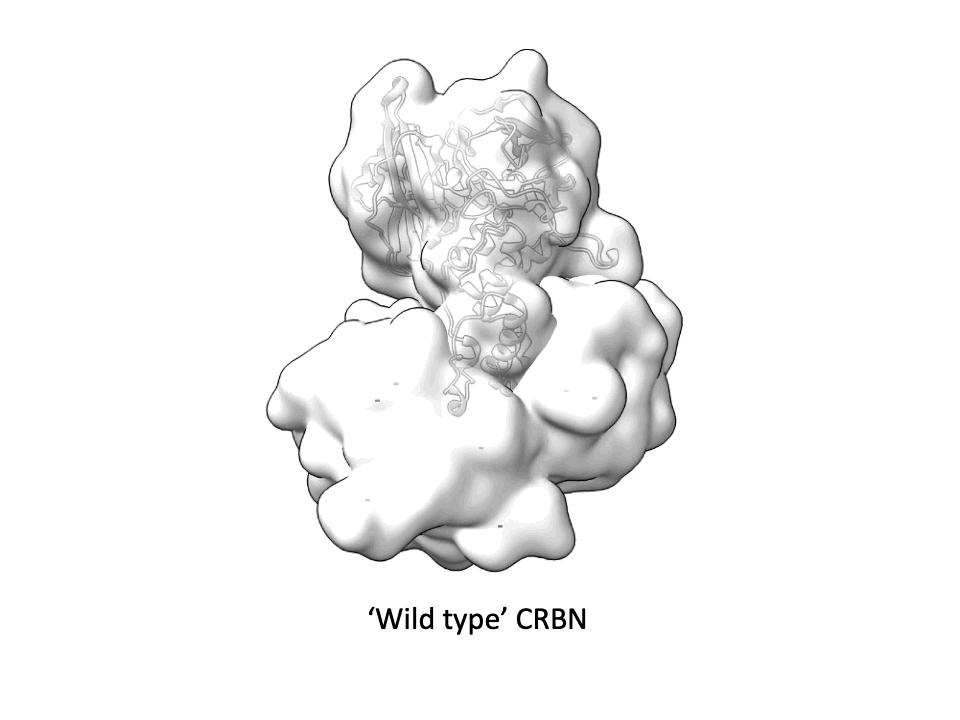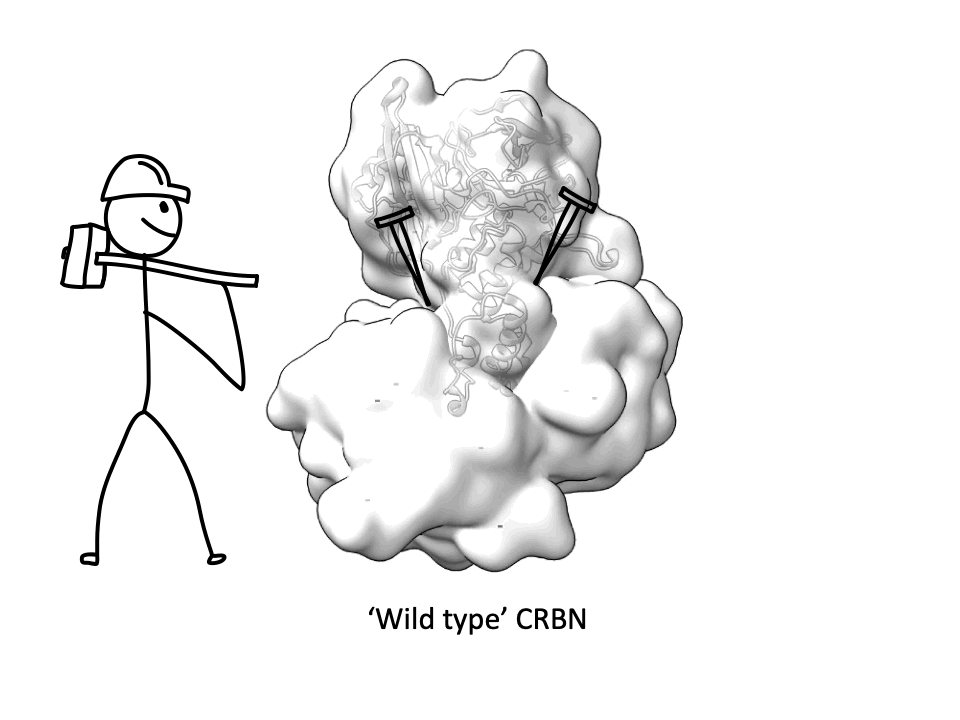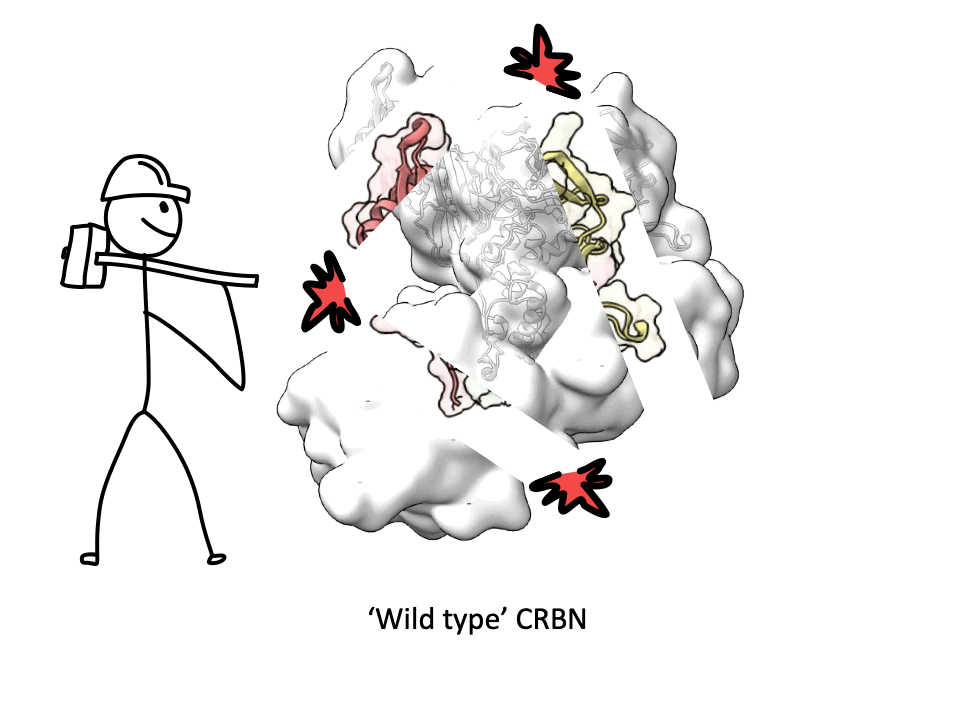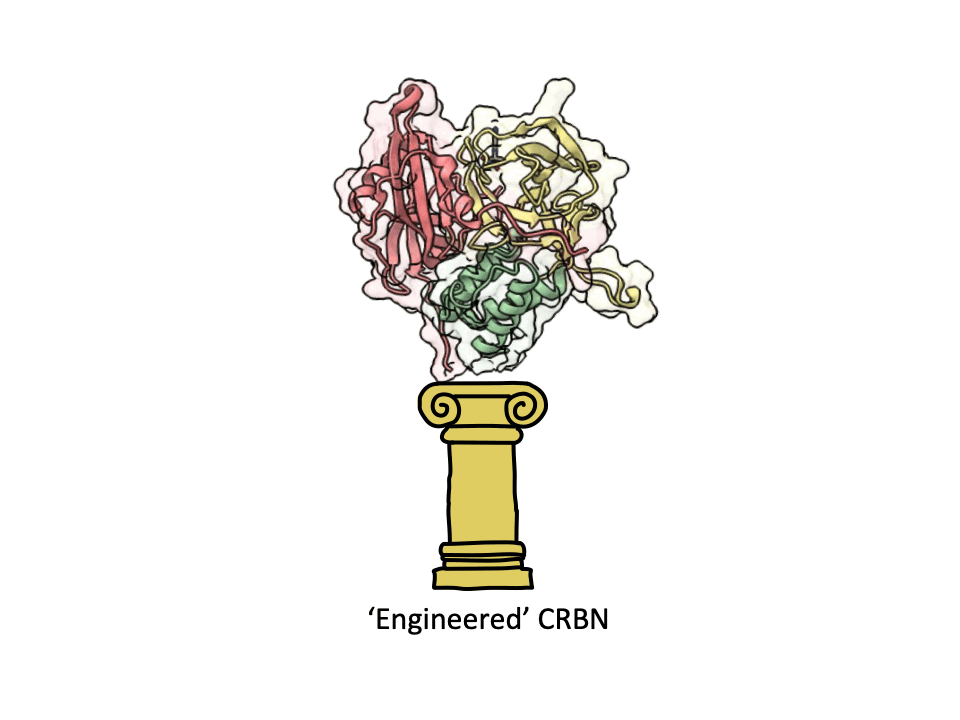
CRBN is one of the most popular E3 ligases in the Targeted Protein Degradation space, but it requires a complex with adapter protein DDB1 to express well. This new soluble CRBN construct enables, high yield expression, rapid screening for novel CRBN binders and can also be used for CRBN interactome assessment with molecular glues. A great collaboration with the Dikic Lab, Enamine Germany, Merck Group, the Langer Lab Max Planck Institute of Biophysics and iBET - Instituto de Biologia Experimental e Tecnológica.
You can read the full article here:
https://www.sciencedirect.com/science/article/pii/S2451945624004604
Animation by PROXIDRUGs team.
We had recently discovered a new signalling cascade in type III CRISPR immune systems, the CalpLTS cascade. In a nutshell, the CalpL protease senses cyclic oligonucleotides that are produced by the type III recognition complex, cleaves the anti-sigma factor CalpT and thereby releases the sigma factor CalpS. In our new study we have discovered that the multi functional CalpL protein also has a ring nuclease activity that degrades the cyclic oligonucleotides. It thus acts as a molecular timer that attenuates the immune response. You can read all the interesting details here.
Congratulations to Thomas Geiger on a new Book Chapter published in Annual Reports in Medicinal Chemistry. Proteolysis targeting chimeras (PROTACs) are hetero-bifunctional molecules that recruit E3 ubiquitin ligases, resulting in polyubiquitination and proteasomal degradation of disease-causing proteins. PROTACs consist of a binder to an E3 ubiquitin ligase connected with a linker to a small molecule, which can recruit protein of interest. Their design makes them modular, and historically majority of the E3 or target binders were reversible small molecules. In recent years, multiple covalent approaches have been utilized in development of PROTAC molecules to leverage potential of covalent molecules to unlock novel E3 ligases as well as drug targets challenging for reversible ligands. The review discusses recent advances in covalent PROTACs, discusses their mechanism of action, with covalency on E3 ligase, target or both and presents key differences in these approaches.
You can read the full article here:
https://authors.elsevier.com/a/1k33PEz97DwLn
A serial switch for kinase activation: Double-phosphorylated Cdk7 turns on full activity! The Cyclin-dependent kinase 7 has two functions, it activates other CDKs to turn on the cell cycle and it phosphorylates RNA polymerase II to initiate transcription. Robert discovered that the first function does not require Cdk7 phosphorylation whereas the second function is fully boosted by the sequential phosphorylation of two sites in the kinase T loop. Read the full story here







